Overview
Neither uterine size, shape or position is permanently fixed. Intermittent myometrial contractions and changes in uterine shape and position are normal during pregnancy. Some of the alterations in the shape of the uterus during pregnancy, such as transient asymmetry related to early gestation (Piskacek uterus) or in the immediate postpartum state, are simply normal variants.
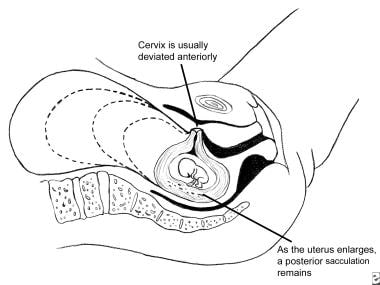
Uncommonly, obstetric complications result from acute or chronic changes in uterine shape or position prior to labor (retroversion or incarceration, prolapse, torsion, herniation or sacculation), during labor (pathologic retractions rings), or postpartum (acute or chronic inversion). [1, 2] The most common uterine malpositioning seen during pregnancy, retroversion with incarceration, is depicted below.
In nonpregnant women, the uterus also assumes numerous positions. Uterine retroversion in nonpregnant women is now recognized as a normal variant that, in most cases, does not result in symptoms. The resort to surgery for the correction of chronic uterine retroversion in the absence of distinct pathologic process (eg, endometriosis, other inflammatory condition) has fallen into appropriate disrepute. This is because of the lack of scientific or experimental evidence supporting the effectiveness of repositioning operations in removing symptoms such as chronic pain or menstrual disturbances or as an isolated treatment for infertility. [3, 4]
Among healthy women, 1 in 5 have a retroverted uterus either as a normal variant of uterine position or as an acquired condition. However, fixed retroversion is not necessarily benign. It can result from important gynecologic pathology. When the uterine contour is distorted by a müllerian anomaly [5] or a strategically placed leiomyoma, or an inflammatory process has occurred in the past (eg, endometriosis or salpingitis with pelvic adhesions), the uterus may become fixed in retroversion/retroflexion and lose its normal mobility. Observational studies have shown a possible association of uterine prolapse with uterine retroversion. [6]
Fixation of the uterus by adhesions is a risk factor for the rare pregnancy complications of uterine torsion, incarceration, or sacculation. Uncommonly during pregnancy, a uterus in retroflexion but without restricting adhesions can also become incarcerated behind the sacral promontory due to a peculiar combination of malpositioning and laxity of the supporting tissues. When uterine retroversion with incarceration develops for any reason during pregnancy, acute symptoms and serious complications are possible, and initial misdiagnosis is frequent.
Most uncommonly, cases of chronic uterine retroversion/incarceration develop uterine sacculation. This is an aneurysmal-like dilatation of the most superior portion of the uterine wall that permits the uterus to enlarge with the consequence of major anatomic distortion. These rare cases can then present at or about term with acute signs and symptoms of maternal or fetal distress, necessitating prompt surgical intervention.
Based on compiled clinical reports and the author's experience, the diagnosis and management of the principal types of both benign and pathologic uterine malpositionings that occur during pregnancy are described in this review.
Uterine Retroversion or Incarceration
Frequency
During early pregnancy, uterine retroversion is a normal positional variant. Typically, first-trimester retroversion is intermittently present 10-20% of the time. However, if retroversion persists into the midtrimester, uterine incarceration is possible, but the likelihood of this complication is low. In only about 2% of uterine retroversions diagnosed during the first trimester does the condition progress to incarceration associated with urinary retention or other acute symptoms. A systematic review found that the incidence of complications, such as premature delivery, need for cesarean section, and poor perinatal outcomes, was lower among cases of uterine incarceration that were diagnosed before 20 weeks of gestation. [7]
Accurate statistics concerning the prevalence of this disorder are not available, a situation true of many infrequently occurring obstetric complications. Most instances of retroversion or incarceration are not reported, and few publications beyond case reports are available for review. A rough estimate is that symptomatic incarceration occurs in 1 per 3000-6000 pregnancies. [8] A retrospective analysis by Utsunomiya et al estimated that uterine incarceration occurs in 1 in 2000 pregnancies, which is higher than the incidences reported in previous studies. [9]
Pathophysiology
Normal pelvic anatomy permits the fundus of the uterus to move relatively freely in the sagittal, vertical, oblique, and anteroposterior planes. In retroversion, the fundus of the uterus is positioned posteriorly. In some instances the uterus is fixed in this position by the presence of adhesions from a prior inflammatory process. Cases of marked retroversion with or without accompanying adhesions may lead to incarceration as the uterus enlarges.
At some critical juncture of uterine size, tissue laxity, and other unknown factors the fundus becomes sufficiently large that it cannot easily exit the hollow of the sacrum and it is incapable of spontaneously rotating anteriorly past the sacral promontory. Because the enlarging uterus cannot rotate anteriorly, it is wedged progressively firmly into the hollow of sacrum while the cervix exerts increasing pressure toward the urethra and/or bladder. Normal voiding eventually becomes difficult or impossible as progressive upward cervical pressure restricts normal funneling of the bladder outlet and obliterates the posterior uterovesical angle. [10, 1, 11, 12]
This condition may recur in subsequent pregnancies. [13] Presumably, the same laxity of supporting structures and the unique features of pelvic anatomy that led to the initial episode are still present and predispose to a recurrence. See the following image.
In unusual cases, the superior wall of the uterus sacculates, permitting the gestation to pass out of the pelvis despite the entrapment. In these rare cases, the pregnancy may advance into the third trimester before the correct diagnosis is established.
Entrapment usually occurs after 12 weeks' gestation. However, it may occur earlier if special conditions such as a multiple gestation or a müllerian anomaly exist, or if a strategically located leiomyoma or adnexal tumor is present.
In nonpregnant women, if retroversion alone is the cause of symptoms, these are usually minimal. Pelvic pain and similar symptoms are principally due to coincidental pathology. Other causes of chronic pelvic pain in which retroversion may be present are discussed below. Evidence to show that isolated retroversion is responsible for abortion or infertility is lacking. When these conditions are encountered, another etiology must be sought. [3]
Gynecologic uterine suspension procedures
Uterine suspension procedures have largely disappeared from gynecologic surgery. Exceptions are cases involving specific cul de sac or adnexal pathology when suspension is performed to help prevent the formation of new adhesions during healing. As an example, uterine suspension is frequently performed when retroversion is associated with endometriosis or tubal pregnancy or when the patient has undergone microsurgical tubal reconstruction procedures to treat infertility.
In nonpregnant women with uterine retroversion and chronic pelvic pain, uterine suspension does not invariably relieve symptoms. This is especially true when patients are monitored long term. The discovery of persisting uterine retroversion alone in an asymptomatic woman is not an indication for prophylactic uterine suspension or a similar procedure. Therefore, clinicians must act conservatively and critically review claims about the relief or lessening of abdominal-pelvic distress after any procedure or therapy to correct retroversion. [14]
Diagnosis
A common clue is a history of progressive difficulty with normal voiding that develops in the early midtrimester. Complaints of pelvic pain and/or pressure or uterine cramping usually accompanies the urinary symptoms. Vaginal spotting may also be present.
In the differential diagnosis, the clinician must consider degeneration of uterine leiomyomata, müllerian anomalies, and various other adnexal or pelvic tumors that either displace a normal uterus into an unusual position or distort its shape. [15] Also to be considered are the rare functional and anatomic malformations of the uterus. These unusual disorders distort the normal uterine contour, and some, such as superior wall sacculation, may be the result of chronic retroversion/incarceration. [16, 17, 18]
In cases of retroversion with incarceration, clinical examination usually yields the following striking findings:
-
Acute anterior angulation of the vagina
-
A cervix abutting firmly against or positioned well behind the pubic symphysis
-
A soft, smooth, nontender mass filling the cul-de-sac
-
An inability to palpate the uterus during abdominal examination or a uterine fundus too small for the known gestational age
In the midtrimester, the initial clinical suspicion is usually easily verified by means of real-time ultrasonography or occasionally by MRI. [19, 20] The posteriorly located uterine fundus occupies the hollow of the sacrum, and the cervix is positioned anteriorly behind the pubic symphysis. Various uterine or adnexal tumors are also revealed during sonography. The possible diagnosis of a müllerian anomaly must also be considered.
Rare mimics of uterine retroversion or incarceration
Several rare conditions can mimic uterine retroversion and incarceration.
With müllerian anomalies, a uterus didelphys or an unconnected rudimentary horn can become positioned in the lower pelvis. This can cause the uterus to rotate or to become displaced anteriorly, mimicking classic retroversion/incarceration.
A posterior uterine sacculation or herniation of the uterine wall can confuse the clinician as the normal anatomy is distorted.
Tumors arising in the adnexa or myometrium can fill the pelvis and force the uterus anteriorly, rotating its position or distorting its shape. This process of tumoral displacement may result in symptoms of pressure or urinary dysfunction similar to those of simple incarceration.
Treatment
Possible therapies for retroversion with incarceration include the following:
-
Bladder decompression by means of intermittent or indwelling catheter drainage
-
Patient positioning (eg, intermittent knee-chest or all-fours positioning, sleeping prone)
-
Manual uterine replacement: manipulation of the uterus into its usual anatomic position, with or without tocolysis and/or anesthesia
-
Colonoscopic manipulation of the uterine fundus with the patient under anesthesia
-
Application of specialized and rarely attempted techniques of replacement (eg, amniocentesis with manipulations or surgical exploration and replacement)
Bladder decompression and patient positioning
The best initial treatment for symptomatic midtrimester incarceration of a normal uterus is a trial of bladder decompression combined with a program of patient positioning. Such management relieves most cases.
At the time of patient presentation, spontaneous voiding may have become impossible and catheterization is usually required. If the bladder is distended, an indwelling catheter is inserted for 24-48 hours. If a urinary tract infection is present, appropriate treatment is initiated.
During the interval of bladder drainage, the patient is instructed to perform repositioning exercises on a frequent basis. These exercises consist of modified all-fours or knee-chest positioning done several times a day for 5-10 minutes, with intermittent maneuvers involving rapid in and out excursions of the abdominal wall. The patient is also instructed to sleep in the prone position. Spontaneous repositioning normally occurs with these steps alone.
Manipulation of the uterus
If the aforementioned outpatient maneuvers prove unsuccessful, manual uterine replacement is considered. After informed consent is obtained and ultrasonography has been performed to verify the normality of the gestation, a tocolytic agent (eg, terbutaline 0.250 mg) is administered subcutaneously 15-20 minutes before reversion is attempted. [21] Note that only anecdotal data address the efficacy of tocolytic therapy in improving the success of transvaginal uterine replacement; therefore, its use is elective. However, tocolytics are generally well tolerated by otherwise-healthy gravidas and, in the opinion of the author, they facilitate the necessary manipulations.
Before the procedure begins, the woman is instructed to void, or, if a Foley catheter was placed, it is fully drained. An attendant is present to physically support and psychologically encourage the patient.
In the replacement procedure, the patient is placed in the knee-chest or all-fours position. An open sided speculum is passed and the anterior lip of the cervix is grasped by using a long Allis clamp or another atraumatic clamp (see image below).
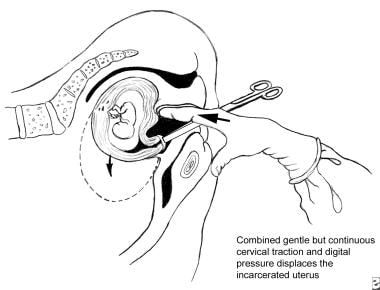 Uterine retroversion-incarceration has occurred. Depicted is the technique of manual uterine replacement with the patient in the knee-chest position.
Uterine retroversion-incarceration has occurred. Depicted is the technique of manual uterine replacement with the patient in the knee-chest position.
The speculum is next removed and the surgeon inserts a finger into the vagina or rectum and applies pressure to the incarcerated fundus while simultaneously applying gentle but constant traction to the cervix in the direction of the introitus. Usually the gravida is instructed to simultaneously draw her abdomen in and out rapidly as the tension is applied. Whether these specific manipulations facilitate uterine replacement is unclear but they do distract her immediate attention and is not harmful. With this combined technique, the uterus should slowly rotate into its normal position. In theory, passing the fundus to either side of the sacral promontory where more room is available is probably best. In the author's experience, such refinements in technique are nearly impossible to achieve, and simple pressure combined with cervical traction suffices.
The maneuvers just described are usually easy to perform. This repositioning maneuver should be neither painful for the gravida nor difficult for the surgeon. Only mild-to-moderate force is appropriate for the replacement. Excessive force jeopardizes patient compliance, and it poses a risk of cervical injury due to the cervical grasping instrument. In addition, excessive force could theoretically damage the pregnancy by acutely distorting the uterus and obstructing uterine blood flow. In unusual instances, when both bladder drainage with repositioning and a trial of gentle manipulation fail to replace the uterus, other methods must be considered.
If simple replacement is not possible, a more involved technique such as the use of anesthesia combined with a tocolytic needs consideration. An epidural anesthetic is best. As an alternative, a general anesthetic could be given with a uterus-relaxing agent to avoid the need for a separate tocolytic drug. However, this approach is not recommended as it is too easy to apply excessive force and easy manipulation of the patient's position is not possible.
To perform uterine replacement when an epidural anesthesia is used, the gravida is initially positioned in lateral recumbency. The epidural anesthetic is administered. Once a proper level is achieved, a speculum is passed and the anterior lip of the cervix is grasped with a long Allis clamp or another atraumatic clamp in the same manner as for the usual uterine manipulation technique. Thereafter, nitroglycerin 0.15-0.5 mg is given intravenously, titrated to effect, or a parenteral beta-mimetic (eg, terbutaline 0.15-0.25 mg administered intravenously or subcutaneously) is administered. Due to its rapidity of onset, short half life, and effectiveness, the authors favor the use of nitroglycerin.
Once the tocolytic has taken effect, cervical traction is combined with rectal pressure against the fundus in the same manner as previously described. Due to the limitations in maternal movement, this is usually performed in the Sim position or in dorsal lithotomy.
Use of real-time sonographic guidance during and after manipulation procedures is prudent. Successful replacement is verified by means of palpation and abdominal scanning.
Specialized and rarely attempted techniques
The literature includes case reports describing numerous techniques for uterine repositioning. These include unusual maneuvers, such as placement of a mercury-filled Voorhees bag in the vagina, along with other more complex methods. In the author's experience, these unusual manipulations are not indicated.
Another technique involves the passage of a colonoscope to provide the upward force necessary to dislodge the entrapped fundus. [22]
In advanced cases, amniotic fluid could be aspirated to reduce uterine volume. This procedure is potentially fraught with problems and cannot be recommended save in the most difficult of circumstances.
Multiple gestations
Multiple gestations, which now often occur in association with assisted reproductive technologies, are special cases. [23] In this setting, the uterus can become incarcerated earlier in gestation than when only a single fetus is present. This is presumably due simply to the increased size of the uterus. If characteristic symptoms occur in women with known multiple gestations—even when the pregnancy has not reached 12 weeks' gestation—retroversion or incarceration should remain in the differential diagnosis.
Of interest, the apparently high incidence of retroversion or incarceration in early multiple gestations might be a new phenomenon due to some unrecognized effect of infertility treatment. Or, past reports of incarceration among spontaneous multiple gestations might have been understated because of sampling or reporting errors.
Postprocedural care
After the procedure, repeat real-time ultrasonography should reveal normal fetal cardiac activity and fetal motion and the patient's original symptoms should resolve promptly. When replacement is successful by any technique, the patient is often instructed to continue to sleep in the prone position, to practice occasional knee-chest or all-fours positioning, or to use a pessary to keep the uterus correctly positioned. These manipulations are probably not necessary but are commonly recommended. No studies have been performed regarding either the efficacy or the necessity of any of these postreplacement treatments.
As a general rule, reincarceration should not recur during the same pregnancy if the uterus has been fully released. The authors have not found these postreplacement treatments necessary; however, because no systematic studies have been performed, the responsible clinician must judge the appropriateness of these several treatments in reference to the specific case in question, as the literature has nothing beyond opinion to offer.
After any procedures to attempt uterine replacement, administration of Rh immunoglobulin is indicated in Rh-negative patients who are not already isoimmunized.
With modern techniques of treatment, serious maternal problems due to simple retroversion or incarceration or consequential fetal complications should be rare. As noted above, in rare cases of chronic uterine retroversion, aneurysmal dilatation of the superior uterine wall (sacculation) may permit the uterus to expand abdominally. [16, 24, 25] If this occurs, the pregnancy may progress even into the third trimester. In such instances, the correct diagnosis is established only when dystocia in labor ensues or when abdominal and/or pelvic examination reveals markedly unusual findings that lead to an MRI study. In the rare case that reaches the third trimester, the uterine malpositioning is fixed, cesarean delivery is required, and major complications are possible.
If treatment is elected, begin with the simple and noninvasive procedures and/or manipulations because they are often successful. The need to administer an anesthetic with a parenteral tocolytic to relieve symptomatic retroversion is, at best, uncommon. In the most unusual of cases, surgical exploration may need consideration, especially if the diagnosis remains uncertain or if the maternal signs and symptoms are severe as important pelvic or adnexal pathology may be present
Persistent retroversion
In a few cases, the uterus remains retroverted and cannot be repositioned despite the administration of tocolysis and the use of regional anesthesia. The best management strategy in this rare setting is moot.
When the uterus is fixed posteriorly and the gestation is in the first trimester, the central issue is whether it is bound down by adhesions due to endometriosis, infection, or previous surgery or immovable due to a tumor or other structural distortion. In the most unusual cases, surgical exploration of the pelvis may be required to determine the cause of the retroversion and, if possible, to return the uterus to its normal position. However, if the cause of the retroversion and/or incarceration is the presence of dense adhesions, surgery may injure the uterus or other pelvic structures, lead to hemorrhage and potentially threaten continuation of the pregnancy. Fortunately, such obstetric and surgical dilemmas are exceedingly rare. In such cases, treatment must be individualized.
As mentioned above, rarely in cases of long standing retroversion/incarceration the uterus balloons into the upper abdomen by sacculation. This process of progressive thinning of the superior segment of the posterior uterine wall initially relieves the compression. [26, 27] Sacculation is not without risk of acute maternal and fetal symptoms and carries a potential for serious complication. Recurrence is rare but possible. [28]
Symptomatic retroversion with bladder outlet obstruction in the puerperium
Symptomatic retroversion with bladder outlet obstruction unusually first occurs in the puerperium. [29] This is because of persisting uterine enlargement or subinvolution combined with flaccidity of the supporting tissues and other unknown causes. These cases are rare and are treated in the same manner as uterine incarceration occurring during pregnancy. Of interest, puerperal problems do not seem to involve the same women who previously developed midtrimester incarceration.
Counseling
The paucity of data concerning the risks associated with retroversion or incarceration during pregnancy makes counseling difficult. Patients should be informed that both symptomatic incarceration and efforts for its relief carry some risk of pregnancy loss.
In the midtrimester, spontaneous abortion may occur due to intrinsic fetal anomalies or because of incarceration alone, or it may follow manual uterine replacement. The risk of loss from uterine replacement procedures is believed to be low. However, the extant literature consists of case reports and opinions and the true risk is not known with any accuracy. Because of this, before a midtrimester repositioning maneuver is performed, a frank discussion with the gravida is essential since incarceration and efforts for its relief carry some risk of pregnancy loss.
Ultrasonography
Because of the general uncertainties, as noted, a real-time sonographic examination and, where appropriate, biochemical tests for fetal normality should precede any intervention. This imaging study is done to reassure both the patient and practitioner, to verify the original diagnosis, to confirm that an anatomically normal and living fetus is present, and to assure that a mass or tumor is not the cause of the incarceration. The study is repeated after repositioning to confirm success of the procedure to verify the presence of an active fetus and normal amniotic fluid.
Allen-Masters syndrome: The pelvic congestion syndrome
In nonpregnant patients, the evaluation of chronic pelvic pain that accompanies uterine retroversion includes a consideration of 2 indistinct and somewhat suspect syndrome complexes: pelvic congestion syndrome (PCS) and Allen-Masters syndrome (AMS). [30, 31, 32]
Allen-Masters syndrome, as originally described in 1955, includes the following 3 elements:
-
History of obstetric pelvic trauma
-
Uterine retroversion with a hypermobile cervix
-
A tear or tears in the posterior serosa and subperitoneal fascia of the broad ligament
Symptoms attributed to AMS are multiple and include chronic pelvic pain, dyspareunia, and various menstrual disturbances.
Although the cause of AMS was originally ascribed to obstetric injuries, a symptom complex similar to that of AMS has also been attributed to endometriosis, independent of a history of obstetric trauma.
The classic approach to AMS consists of first visually establishing the diagnosis during laparotomy or laparoscopy. Any demonstrated peritoneal broad ligament defects are then either fulgurated or sutured.
Other surgical procedures have traditionally been performed at the same time. These procedures include shortening of the uterosacral ligaments, resection or obliteration of the pouch of Douglas, or other similar operations to cause the uterus to remain anteflexed.
In theory, repair of the tears in the posterior serosa and subperitoneal fascia of the broad ligament, along with the performance of ancillary procedures (eg, plication of the uterosacral ligament to return the uterus to the usual anterior position), are curative. This is a problematic syndrome with the pathophysiology ill defined. AMS remains a diagnosis of exclusion. [33]
Pelvic congestion (Taylor) syndrome
Another possible diagnosis is the pelvic congestion syndrome (PCS), or Taylor syndrome. [33] This condition is characterized by menometrorrhagia and symptoms of continuous pelvic pain. Upon examination, the uterus is variably enlarged and soft, and some degree of tenderness is present. Uterine retroversion is again a common but not invariable finding. The cervix may be patulous or cyanotic. Among other treatment possibilities, both hysterectomy and vascular embolization have been used to manage PCS. The symptoms of this condition are nonspecific and poorly defined. Both this and the AMS are suspect diagnostic entities.
Uterine Torsion
Frequency
Uterine torsion is sporadically reported in association with human medicine. [34, 35, 36, 37, 38, 39, 40, 41, 42]
Most reported cases appear in the veterinary literature. The prevalence of torsion is not known but is at best rare. The condition is potentially serious, the pathophysiology is poorly understood, and the appropriate management in early gestation is not established. The earliest reported age for uterine torsion during pregnancy is the 6th gestational week; the latest is the 43rd week.
The rare cases of torsion that present at or about term are almost invariably discovered during the first stage of labor as a result of acute maternal or fetal distress that leads to an exploratory surgery. [43] As the symptom complex is nonspecific, and as surgical exploration is normally required to establish the correct diagnosis, intervention is frequently delayed, increasing the risk of complications. [44]
Torsion rarely occurs in the non pregnant state. The presentation is often nonspecific, and most cases involve distinct pathology. [45]
Table 1. Presenting Signs and Symptoms of Uterine Torsion According to the Degree of Uterine Rotation [46] (Open Table in a new window)
Uterine Torsion, degrees (n = 212) |
Signs and Symptoms* |
||||||
Intestinal |
Urinary |
Pain |
Shock (Hemorrhage) |
Labor Dystocia |
Other† |
None |
|
≤90 (n = 66) |
10 |
5 |
43 |
4 (6) |
7 |
13 |
9 |
>90 to 180 (n = 122) |
17 |
|
91 |
22 (13) |
19 |
19 |
14 |
>180 to 360 (n = 14) |
7 |
10 |
14 |
6 (1) |
3 |
3 |
0 |
>360 (n = 6) |
0 |
0 |
6 |
6 (1) |
6 |
6 |
0 |
Unknown (n = 4) |
0 |
0 |
4 |
2 (0) |
0 |
0 |
0 |
*Some cases include more than 1 sign or symptom. † Hypertonic uterus, PROM, pre-eclampsia, uterine rupture, etc |
|||||||
Pathophysiology
In a uterine torsion, the uterus twists more than 45° around its long axis at the junction between the cervix and the corpus. The extent of the torsion is most often 180°. However, cases involving twists of 60-720° have also been described.
When a torsion is found, dextrorotation of the uterus is the most common finding, following the normal orientation of the myometrial fibers. Symptomatic torsion occurs when the degree of twisting is sufficient to interfere with arterial or venous circulation. In most instances, the cause of uterine torsion is obscure and no specific pathology is identified. Structural abnormalities of the uterus or adnexa are, however, demonstrated in at least 20% of cases. Vascular obstruction can lead to an acute restriction in blood flow or predisposition to abruptio placentae.
In theory, the acute uterine positioning causes direct compression of the uterine veins and perhaps the ovarian veins, resulting in acute maternal symptoms. The torsion can also threaten fetal survival. As venous outflow is obstructed vascular pressure within the placental cotyledons is acutely increased. This is theorized to predispose to placental separation (abruptio placentae). Whether this mechanism is correct is speculative. Regardless of the exact pathophysiology, an association between abruption and episodes of acute torsion is noted.
Entities reportedly associated with torsion include the following:
-
Abnormal fetal presentation (eg, a transverse lie)
-
Distortion in uterine shape due to uterine leiomyomas
-
Müllerian anomalies
-
Pelvic adhesions
-
Large ovarian neoplasms that distort the shape or position of the uterus
-
A long or rigid cervix or a weakness at the junction of the cervix and uterine corpus
-
External cephalic version procedures
-
Sudden maternal movements, as may occur during automobile accidents or, most rarely, even during normal physical activity
-
Abnormal pelvic architecture
-
Hydramnios
-
Multiple gestation
-
Hyperactive fetus
-
Interstitial pregnancy
-
Unknown and/or idiopathic factors
Although many of these listed associations and predispositions are of frequent occurrence during pregnancy, torsion is rare. This suggests that some unique concatenation of features are needed for symptomatic torsion to occur. Drawing from prior reports, factors may include certain maternal movements, postures, or positions; irregular contractions of the abdominal muscles; changes in myometrial tone; functional variations in the size, position, and mobility of the bladder and rectum; fetal movements; and possibly even segmental uterine contractions.
Although innovative theories of etiology are noted, many of the identified predisposing factors are common in the obstetric population, the condition remains rare, and no convincing pathophysiologic explanation for most cases has been offered.
Clinical presentation
Although uterine torsion can be asymptomatic, most women undergoing a torsion present with symptoms. The range of the associated symptoms is both wide and nonspecific, mimicking those of many much more common conditions. [47]
Common presenting symptoms include abdominal pain of acute onset, nonspecific GI distress, and urinary dysfunction. GI symptoms include nausea, vomiting, diarrhea, abdominal distention, and tenderness. Urinary symptoms can include urgency, frequency, nocturia, oliguria, and hematuria. Other reported symptoms are vaginal bleeding, hypertonic uterine contractions, or premature rupture of membranes. Acute symptoms which are much less frequently encountered include maternal cardiovascular collapse (syncope) or abruptio placentae. If the torsion occurs at term, obstructed labor is possible, or an acutely abnormal electronic fetal monitoring heart rate pattern may develop.
Diagnosis
The clinical challenge of uterine torsion lies in its elusive diagnosis. In most case reports, the correct diagnosis was rarely established before surgical exploration. Torsion is also rarely asymptomatic. [48] Recently, both MRI and CT scanning have been used to help make the diagnosis prior to exploratory surgery. [45, 49, 50]
Jensen described several clinical findings characteristic of uterine torsion, including the following: [46]
-
Upon surgical exploration, uterine rotation about the vertical axis is observed in association with marked venous engorgement and edema of the parametrial tissues.
-
Upon abdominal examination, the round ligament can be palpated anteriorly, stretching across the maternal abdomen
-
Upon pelvic examination, the uterine artery is palpable, pulsating anteriorly.
-
Upon speculum examination, the vagina and/or the cervical canal is distorted.
Mistaking torsion for a nonsurgical entity or another medically managed obstetric complication is potentially disastrous. Exploration is usually performed to investigate acute maternal distress with or without a nonreassuring fetal heart-rate pattern, conditions that permit little time for extensive evaluation. Common prompts to expedient surgery are maternal symptoms suggesting bowel obstruction, uterine rupture, acutely obstructed labor or abruptio placentae.
Potentially serious sequelae are possible from uterine torsion. In his literature review, Jensen reported 19 maternal losses from torsion. He identified as important risk factors the degree of torsion (>180º) and the period of gestation (>20 wk). [46] The same risk factors were also identified by Piot. [51] These data, limited as they have been derived over many years, provide a rough indication of the high-risk cases. Although maternal mortality from this condition is now rare in modern practice, Guie has reported a case ending in maternal death, and Cook a case in which both ovaries and uterus were necrotic and the mother in shock at the time of exploration, reminding clinicians of the potential risk. [34, 44]
When one considers these data, one must remember that the statistics were derived by the compilation of case reports transpiring over years at many different institutions. Therefore, these reported risks are almost certainly not representative of maternal risks in modern, fully equipped hospitals with current diagnostic equipment. Nonetheless, these data are a reminder that serious sequelae of torsion are possible, especially as the correct diagnosis is often delayed.
Uterine torsion is a rare obstetric complication. Nonetheless, because of its associated risks, torsion should be included in the differential diagnosis when severe but nonspecific abdominal pain occurs during pregnancy. Torsion is also a consideration, albeit a remote one, when dystocia is present or when acute fetal jeopardy develops during labor.
Treatment
Given the unique circumstances, establishing standard guidelines for management at the time of surgical exploration is impossible. However, several management points should be considered. At exploration, any contributing pathology of the uterus or adnexa is best removed. Whether the pregnancy should continue is the central question. This depends on the gestational age and the clinical setting. The risk of repeat torsion or other potentially serious complications is simply not known. Also uncertain are whether any procedures should be performed to fix the uterus in its usual anatomic position at the time of surgery and, if so, which procedure to attempt.
It seems reasonable to base the treatment of torsion on gestational age. When torsion is discovered during exploratory surgery before the period of presumed fetal survival (ie, before 23-24 wk), promptly returning the uterus to the normal position without conducting a delivery is best. In these cases the uterus should only be emptied if the torsion cannot be relieved or if other serious pathology is present that precludes allowing the pregnancy to continue.
In the various clinical case reports, especially if the uterus was not emptied, plication of the round ligaments was commonly performed. Although no systemic studies have been conducted, if delivery is not accomplished, a uterus-stabilizing procedure such as a plication seems prudent. The benefit of plication is not established but acute symptomatic torsion is so rare and difficult of diagnosis that no random study is possible. Thus, in this unique setting, because round ligament plication procedures for uterine stabilization are probably risk free and because they may be beneficial, they are recommended. In continuing pregnancies, steroids should be administered after the 24th or 25th week of gestation.
In the interval between the limit of fetal viability at 23-24 weeks' gestation and the 34th week, or in the rare instance when the diagnosis is securely established by MRI or other study before a laparotomy and signs and symptoms are not compelling, best management is unclear. If the abdomen has been opened and the uterus successfully rotated into the anatomic position, apparently relieving the torsion the surgeon must balance the unknown risk of a maternal and/or fetal complication if the delivery is not accomplished against the immediate risk of substantial prematurity.
When the fetus is beyond the 34th week of gestation at the time of diagnosis, the best approach at the time of the original laparotomy procedure is prompt cesarean delivery.
At exploration, the surgical treatment may prove complicated. In several reported cases of torsion, the degree of rotation was so severe that detorsion was not possible. The hysterotomy incision eventually made at the time of cesarean delivery could only be performed on the posterior uterine wall. Some surgeons described these posterior incisions as inadvertent, whereas others deliberately performed them when efforts to rotate the uterus to its normal position proved unsuccessful. Numerous case reports relate that the uterus could not be rotated into the normal position until it was emptied.
Too few reports are available to permit accurate assessment of the long-term sequelae of delivery through a posterior hysterotomy incision. When the diagnosis is made at surgery, it seems reasonable to first attempt to rotate the uterus into the normal position before performing the myometrial incision. If detorsion is impossible, the posterior surgical approach is used. A transverse incision is best. It is curved upward at the ends in a manner mimicking that of the usual anterior-wall procedure.
In a number of case reports of torsion, prophylactic plication of the round ligaments by various techniques was performed after delivery of the fetus. The intent in these procedures was to stabilize the uterus to prevent the recurrence of torsion in the puerperium. The effectiveness and the necessity of this treatment is unknown.
In summary of the extant literature, if uterine torsion is discovered during an exploratory laparotomy, the surgeon should initially search for a pathologic process responsible for the malrotation (eg, uterine distortion due to leiomyomas). None may be found.
If a pathologic process is present it should be removed, if technically possible. If the period of gestation is sufficiently advanced and delivery is decided on, the type of myometrial incision depends on uterine position and needs to be individualized because derotation of the uterus may not be possible until the products of conception are removed. A posterior-wall hysterotomy may well be required. An elective uterine fixation procedure is elective after the baby is delivered.
In contrast, a fixation operation should be performed to help prevent a recurrence if delivery is not accomplished at the time of the original exploration. Both the etiology of this peculiar and rare disorder is speculative and the risk of retorsion in the same or a subsequent pregnancy remains unknown.
Uterine Herniation
Frequency
Herniation of the uterus through an abdominal wall defect (eg, an incisional or umbilical hernia) is a very uncommon condition in Western practice. [52] Most cases are reported from the nonindustralized world. [53, 54, 55, 56] According to Saha, fewer than 20 cases have been reported in the medical literature. [57] The most common associations for anterior uterine herniation are prior maternal abdominal wall defects, most often from prior surgery or advanced diastasis recti associated with advanced multiparity.
Presentation
The clinical presentation is striking. A history of prior abdominal surgery or cesarean delivery via a midline incision or a prior, unrepaired umbilical hernia is noted. Beginning in the midtrimester when the uterus is sufficiently large so as to become an abdominal organ, it progressively prolapses anteriorly into the hernia sac. [58]
Varying degrees of this condition are observed. In the most extreme cases, the fundus of the uterus completely passes between the rectus abdominis muscles and, with time, beyond the confines of the abdominal wall. This results in a mass (uterus, peritoneum, skin) that extends outward at virtually a right angle from the maternal abdomen. The marked protrusion of the abdomen in a number of these cases is remarkable.
Pathophysiology
The problem arises from marked attenuation of the abdominal wall with advanced diastasis recti accompanied by weakening of the fascia. Numerous potentially serious complications, including spontaneous abortion, strangulation/incarceration, abruptio placentae, uterine rupture, and intrauterine death, have been reported in association with extreme cases of uterine herniation. Uncommonly, the constant pressure of the uterus against the hernia sac results in ulceration of the overlying skin.
Diagnosis
The diagnosis is confirmed by a combination of simple palpation and real-time ultrasonography scanning. Usually the markedly attenuated peritoneal/fascial/skin covering of the uterus permits easy palpation of the round ligaments and of the normal irregularities of the surface of the gravid uterus. Presumably, a pelvic or abdominal tumor might result in some confusion to the clinician if the case history is not known. Simple real-time ultrasonography scanning promptly verifies the correct diagnosis for the prolapsed mass.
Treatment
As potentially serious complications are possible with this disorder, if possible, the uterus should be reduced through the hernia. In advanced cases, replacement may prove impossible due to size of the uterus, prolonged anteflexion, and resultant maternal discomfort. Activity restriction, occasionally abdominal binding antepartum or intrapartum, and close observation are the mainstays of management.
Herniorrhaphy has been performed during pregnancy but definitive repair of the abdominal wall defect is normally conducted only following delivery due to the risks of wound disruption and the constant pressure of the underlying uterus. The abdominal wall is often so attenuated that an extensive repair with a permanent mesh is required. Vaginal delivery is possible if the uterus can be returned to the abdominal cavity and maintained in the normal anatomic position. As this condition is so rare, treatment must be individualized.
Functional Uterine Malformations During Pregnancy
Frequency
The functional uterine malformations are a group of unusual disorders that constitute transitory outpouchings or distortions in the wall of a pregnant uterus which disappear following delivery. [1] True full wall uterine dilatations (sacculations) are to be distinguished from the frequently noted asymmetric enlargement of a normal uterus in early pregnancy due to an implantation in one cornua (Piskacek uterus, grossesse angulaire), pathologic retraction rings, and the occasionally appreciated mild and temporary asymmetry of the postpartum uterus believed to be due to differential tonus of the muscles making up the left and right fused müllerian tubes (pseudo uterus arcuatus).
No reliable data concerning the prevalence of these conditions are known. Some types of functional uterine disorders such as a Piskacek uterus are frequent and little commented on, even in standard textbooks of obstetrics. Other functional disorders such as pathologic uterine sacculation are exceedingly uncommon or rare. For the latter conditions, the available literature consists of case reports with data collected from many years of clinical observation. Thus, accurate estimates of occurrence are difficult, and best management is hard to establish.
Pathophysiology
In true or pathologic uterine sacculation, dilatation of the myometrium includes all layers of the uterine wall, with the outer layer of the myometrium continuous with the normal myometrium of the remaining uterine corpus.
Possible causes for sacculation include myometrial herniations along the path of blood vessels, müllerian fusion defects, poorly healed myometrial incisions, or areas of the myometrium injured by prior curettage or other procedures, wall weakness arising from exaggerated trophoblastic invasion, or distortion from leiomyomas or müllerian anomalies.
Most often, pathologic sacculation is associated with chronic retroversion/incarceration or other types of uterine entrapment. Sacculation constitutes the mechanism by which the uterus continues to enlarge by progressively assuming a different shape. In these rare instances, thinning and aneurysmal-like enlargement of the portion of the myometrium that is not incarcerated slowly develops, resulting in unusual distortions of the uterus that are normally incompatible with vaginal delivery.
The most bizarre type of true sacculation is localized ballooning of the uterine wall that is not associated with uterine entrapment or deformity. In these cases, the cervix is normally positioned and the sacculation is an incidental discovery at the time of cesarean delivery. This peculiar type of localized sacculation is most often but not invariably at the placental implantation site. The etiology is unclear but may represent a hormonally induced localized myometrial weakness.
A pathologic uterine retraction ring (Bandl ring) is another transient alteration in uterine shape of obstetric importance. [59] Such pathologic rings form in advanced cases of obstructed labor from the normal retraction ring that marks the separation between the upper, thick, and contractile portion of the uterus and the lower, thinner segment. In situations of obstructed labor, the physiologic ring becomes thicker and stronger as effacement progressively thins the lower segment and the upper muscular portion of the uterus increases in size. At some point, the ring itself becomes an obstruction, blocking descent. In some cases, the ring may directly contribute to fetal injury. Classical teaching is that the presence of a pathologic retraction ring is an indication of marked dystocia and impending uterine rupture.
Uterine diverticula are anatomic as opposed to functional uterine malformations. The term is usually applied only to permanent bulges or defects demonstrated in the nonpregnant uterus.
These exceedingly rare lesions are usually small and asymptomatic. They may be incidental findings noted at the time of surgical exploration. The cause is presumed to be a localized myometrial defect due to some type of prior myometrial injury. Best management is local excision with closure of the uterus in layers. Subsequent management is, again, individualized.
No systemic investigations of these unusual conditions exist and there is much confusion in the literature concerning nomenclature and possible etiologies.
Diagnosis
A Piskacek uterus is a normal, benign, and minor variant of sacculation involving uterine asymmetry of the corpus. A degree of uterine asymmetry can frequently be palpated in thin individuals in the first trimester of pregnancy. The Piskacek uterus needs to be distinguished from interstitial pregnancy. The latter involves an implantation high in the uterine cavity with a ballooning of the interstitial area and marked thinning of the overlying myometrium. Ultrasonography scanning can easily distinguish between these conditions. The Piskacek uterus is an asymptomatic transient (< 10 wk) normal variant of uterine shape. An interstitial pregnancy commonly occurs at a later gestational age, has characteristic ultrasonography findings, is usually associated with maternal symptoms, and carries a substantial risk of rupture/hemorrhage.
The classic finding in a Bandl ring is an hourglass deformity of the uterus that may either be palpated abdominally or noted at the time of cesarean delivery. When a pathologic ring forms, it may constitute so intense a constriction as to require surgical excision and/or pharmacologic uterine relaxation for its relief. Pathologic rings are usually thought of in singleton pregnancies characterized by prolonged labors but can also occur in twin gestation, leading to difficulty with descent or delivery of the second twin.
Pathologic sacculations are usually diagnosed at the time of surgical exploration for acute signs and symptoms suggestive of maternal or fetal distress. Most instances of sacculation are associated with chronic uterine incarceration. The combination of a small for gestational age uterus, a presenting part deep in the cul de sac, and a cervix that is either not palpable or markedly deviated anteriorly or posteriorly may suggest the correct diagnosis. [24] MRI has also been reported to assist in establishing the actual anatomic position of the uterus. [19] In most cases of pathologic sacculation, vaginal delivery is impossible because the uterine distortions result in the external os being positioned above the public symphysis. In these cases, labor is contraindicated. Although exceedingly rare, pathologic sacculation has been reported to recur in subsequent pregnancies.
At the time of surgery, the anatomy is frequently so distorted that the usual site of cesarean entry is either not identified correctly or cannot be reached. The anatomic site of the myometrial entry may not be apparent until after removal of the fetus and placenta and the uterus cannot normally be returned to the normal anatomic position until the products of conception have been removed and uterotonics administered. These cases are so uncommon that the surgical approach needs to be individualized.
Uterine Inversion
Frequency
Uterine inversion is an infrequent and potentially dangerous obstetric complication. [56, 60, 61, 62] It is reported to occur at a rate of 1 case per 2000-23,000 deliveries. This wide range reflects differences in recording methods in patient populations and perhaps in routine obstetric techniques. As examples of recent literature, in the 24-year series reported by Baskett involving 125,081 deliveries, the incidence was 1 in 3737 for vaginal deliveries and 1 in 1860 for cesarean deliveries. [63] In the series described by Hussain et al [64] and Shah-Hosseini et al [65] , puerperal inversions occurred in 1 in 1584 and 1 in 4345 deliveries, respectively, while Abouleish [66] reported an incidence of 1 in 3643. A study by Coad et al reported that the incidence of puerperal uterine inversion was 2.9 per 10,000 among 8,294,279 deliveries in a sample cohort from 2004-2013. 37.7% of the women with a uterine inversion had an associated postpartum hemorrhage, 22.4% had a blood transfusion, 6.0% required surgical management and 2.8% had a hysterectomy. [67]
So few reports describe gynecologic inversion that its incidence cannot be estimated accurately. Rare nonobstetric inversion usually occurs due to prolapsing uterine tumors. [68]
Uterine inversion may occur in the immediate postpartum period or, less frequently, during the puerperium. Inversions are usually described as recent/acute (< 30 d after delivery) or chronic (>30 d after delivery). Within the category of recent inversion, a distinction is made between acute and subacute varieties. Acute inversions occur within the first 24 hours after delivery, whereas inversions occurring more than 24 hours after delivery but before the 30th postpartum day are termed subacute.
Pathophysiology
The underlying pathophysiologic mechanism for uterine inversion is unknown. Several clinical observations and associations are pertinent. Clinically, the principal factors that predispose to puerperal inversion are a fundally implanted placenta, flaccidity of the myometrium around the implantation site, and a dilated, immediately postpartum cervix. In some cases, the presence of a short cord and/or mismanagement of the third stage with imprudent cord traction undoubtedly contribute to inversion. However, the classic claim of meddlesome midwifery as the cause of inversion is insufficient to explain all reported cases, and it may not be a factor in most. Some inversions have occurred without cord traction and may even occur at cesarean delivery via the myometrial incision after the neonate is born but before the placenta is removed. [69] This process has once surprised the author. An inversion during a cesarean may happen spontaneously or after the administration of a potent tocolytic, such as nitroglycerin. [70] In these and other cases of acute inversion, the flaccidity of the upper portion of the myometrium is striking. If the uterus remains flaccid in the moments immediately after parturition and if the placenta is fundally implanted, downward protrusion of the fundus is possible and probably frequent. Under unique circumstances, this myometrial flaccidity (perhaps sometimes aided at this critical juncture by classic inappropriate cord traction) permits the fundus to indent with or without the placenta remaining implanted. This results in a situation where inversion becomes possible.
To result in an inversion, the uterus must resume contracting at precisely the right moment to force the previously inverted fundus or fundus-placental mass downward, driving it deep into the lower uterine segment. If the cervix is sufficiently dilated and the force of contraction sufficiently strong, the myometrial/placental mass can be squeezed through it, resulting in complete inversion. In situations less extreme than that just described, the indented fundal wall is itself trapped within the uterine cavity, resulting in a partial inversion.
In complete inversions once the fundus passes through the cervix, the cervical tissues function as a constricting band and edema rapidly forms. The prolapsed mass then progressively enlarges and increasingly obstructs venous and finally arterial flow, contributing to the edema. Thus, replacement of the uterus becomes more difficult the longer the inversion persists. In chronic or neglected cases, serious tissue injury or necrosis are possible, albeit rare, sequelae.
When considering the pathophysiology of inversion, one should wonder why it does not happen with greater frequency than it does. Postpartum uterine flaccidity, fundal placental implantation, and some degree of cord traction are ubiquitous. However, inversion is so infrequent a complication that these otherwise common factors must act in concert in a unique fashion to permit it to occur. [71]
Experience with the protocol for active management of the third stage of labor (AML) emphasizes the importance of uterine tonus immediately after the delivery of the baby in the etiology of inversion. An unanticipated benefit of AML is that the incidence of uterine inversion is markedly reduced. This is true despite the routine use of immediate cord traction after delivery of the neonate, part of the AML protocol but a technique that might be thought to increase the risk of inversion. [63]
The important difference in the AML protocol versus natural birth is the routine administration of intravenous oxytocin immediately after the neonate is delivered but before signs of placental separation appear. [71] The oxytocin apparently maintains myometrial tone, and it is presumed that this effect reduces the risk of an inversion. Thus, it seems fair to conclude that the maintenance of uterine tonus is an important factor in preventing inversion. [72]
Terminology for the severity of an inversion is based on 2 clinical features: (1) the extent of prolapse in relation to the cervix and (2) how far down the birth canal the resultant mass extends (see Table 2).
Table 2. Degrees of Uterine Inversion (Open Table in a new window)
Degree |
Definition |
First |
Inverted uterine wall extends to but not through the cervix |
Second |
Inverted uterine wall protrudes through the cervix but remains in the vagina |
Third |
Inverted uterine fundus extends outside the vagina |
Fourth |
Total inversion; both the vagina and uterus are inverted |
Clinical Presentation / Diagnosis
Once the correct diagnosis is made, the surgeon should act with celerity. Uterine replacement may be surprisingly easy when performed early. An immediate procedure may avoid the resort to the various complex manipulations to return the uterus to its correct anatomic position. As discussed below, a potential exception to this rule of immediate replacement is the situation of inversion when the placenta remains attached to the uterus .
The diagnosis of uterine inversion is established based on clinical signs and symptoms, except in unusual circumstances such as the rare chronic cases. Classic observations include early-onset postpartum hemorrhage and the sudden appearance of a vaginal mass followed by various degrees of maternal cardiovascular collapse. In incomplete inversions a mass is may note be seen externally and postpartum hemorrhage is usually the most striking symptom. The differential diagnosis depends upon the rapid exclusion of a number of other possible obstetric complications. But, with a combination of an initial physical examination and bedside realtime ultrasound scanning , the correct diagnosis is rapidly established. In some instances the rapidity of the maternal blood loss may necessitate prompt surgical exploration without a completed evaluation at which time the correct diagnosis will be obvious.
The following entities are included in the differential diagnosis of uterine inversion:
-
Prolapse of a uterine tumor or a large cervical polyp
-
Passage of previously unsuspected second twin, secundines, or a succenturiate lobe
-
Gestational trophoblastic disease
-
Occult laceration of the genital tract with uterine atony
-
Severe uterine atony
-
Uterine rupture
In the classic case, the initial and ultimately diagnostic event is the sudden and disconcerting protrusion of a large, dark-red, polypoid mass through the vagina either accompanying or immediately following the delivery of the placenta. The characteristic appearance of the inverted uterus protruding externally is both surprising and startling and immediately establishes the correct diagnosis. In approximately 60-70% of cases, the placenta remains attached (at least initially) at the moment of inversion.
As a general rule, the extent of the observed bleeding varies depending on the degree of prolapse. Depending upon the series reported, in about half of all complete inversions, some degree of acute maternal cardiovascular decompensation occurs. A characteristic comment in case reports is that the extent of shock is more than that attributable to the observed blood loss alone.
A theory to explain this observation is that stretching of the broad ligament or compression of the ovaries as they are drawn together results in a parasympathetic reflex, which contributes to the acute symptoms (neurogenic shock). However, estimated blood losses at the time of delivery or of any obstetric hemorrhage are notoriously inexact. Therefore, the apparently sudden onset and often unanticipated shock may be simply an artifact of observation and inaccurate estimation of the actual volume of the associated hemorrhage.
Whatever the mechanism, ie, simple blood loss, neurogenic reflex, or a combination thereof, prompt uterine replacement combined with vigorous fluid resuscitation including blood transfusion, as required , will reverse the maternal hypotension.
With partial or Second Degree inversions in which the fundus passes through the cervix but does not appear externally, the findings from the initial manual examination may be misleading and suggest a uterine or pelvic tumor. On abdominopelvic examination, as globular and somewhat irregular, central pelvic mass approximates the size of a 22- to 24-week pregnancy is palpable. This mass consists of the adnexa compressed together in the midline along with the partially inverted fundus, which is itself entrapped in the lower segment of the uterus. The most obvious diagnostic clue is the clinician's inability to palpate the uterine fundus during abdominal palpation or the inability to visualize or palpate the cervix during pelvic examination.
Unfortunately, inability to locate the cervix can have other causes. In simple severe atony, heavy bleeding may preclude observation of the cervix. Alternatively, the uterus may be so flaccid that it is difficult to palpate, especially-- as is common in current practice -- if the mother is obese. Therefore, neither nonvisualization of the cervix nor the inability to palpate the uterine fundus can be considered as pathognomonic signs of inversion.
If the inversion has not passed through the cervix into the birth canal (First Degree) and is retained entirely within the uterus , establishing the correct diagnosis is even more difficult. Although hemorrhage may be present, the cervix is palpable and it usually can be visualized. Fortunately, such partial inversions are very uncommon. Even in this situation, findings from abdominal palpation are strikingly atypical, and the correct diagnosis is easily and rapidly established by means of bedside real-time sonographic examination. [49]
In instances where the accompanying hemorrhage or shock is sufficiently alarming to prompt immediate surgical exploration, the correct diagnosis of an incomplete inversion may be established only at the time of laparotomy. [73]
Chronic uterine inversion
Chronic inversion is a rara avis indeed. [74] The literature reports cases discovered as late as 14 weeks' postpartum. This condition manifests with a variety of vague symptoms, including persistent vaginal bleeding or discharge, as well as symptoms of low back pain or pelvic pressure. Such symptoms may also be accompanied by malaise or low-grade temperature elevation. At least once case was complicated by a mesenteric vein thrombosis. [61] Again, the pathophysiology is not understood. This diagnosis is difficult to establish on clinical grounds alone. Here again, real-time ultrasonography is especially helpful. [49] Ultrasound findings that strongly suggest the correct diagnosis include a large, homogeneous mass apparently contiguous with the uterus; the inability to identify a normal appearing fundus; and a peculiar, globular uterine contour.Most of these late cases will require surgical procedures for replacement.
Treatment: General Principles
Rapid diagnosis and aggressive management of uterine inversion minimizes the principal risks of acute inversion which include hemorrhage and related cardiovascular collapse or shock. [75] Cardiovascular supoort, the recruitment of assistance, and moving promptly to an operating room for a prompt attempt at replacement are the basics of best management. The approach to treatment should follow a logical progression.Transvaginal replacement is first attempted under parenteral tocolysis. If this does not succeed then the administration of anesthesia combined with a surgical procedure will be required. The properly timed administration of uterotonics and attentive post replacement care to assure continued normal uterine position are also of importance.
Manage shock
Summon nursing assistance and another surgeon.
Begin fluid resuscitation with 2 large-bore intravenous lines. Promptly administer 1 L or more of a balanced isotonic salt solution ( e.g. Ringer's lactate).
Submit specimens to the laboratory to prepare for possible transfusion of blood or blood products. Also send samples to determine baseline values of the following:
-
Hemoglobin concentration
-
Hematocrit
-
Coagulation factors (eg, prothrombin time, activated partial thromboplastin and prothrombin time, fibrinogen concentration, fibrin split products )
Insert a Foley catheter.
Immediately summon an anesthesiologist.
Treat aggressively
Administer oxygen.
Order the appropriate surgical equipment, and instruct assistants to prepare the operating room for possible laparotomy.
Once in the operating room, administer tocolytics to promote uterine relaxation. These drugs may include one of following:
-
Nitroglycerin 0.250-0.500 mg given intravenously over 1-2 minutes (preferred)
-
Terbutaline 0.100-0.250 mg given slowly intravenously over 1-2 minutes
-
Magnesium sulfate 4-6 g administered intravenously over 20 minutes (not recommended as initial treatment)
Attempt prompt replacement of the uterus.
-
Begin with a trial of manual replacement per vagina. Conducted with adequate uterine relaxation, this maneuver is highly likely to be successful.
-
If manual replacement fails, promptly perform a laparotomy for surgical replacement unless the clinician is trained in one of the vaginal surgical approaches. During laparotomy, general anesthesia with a uterine-relaxing agent is suggested, especially if a parenteral tocolytic was not previously given or if relaxation is inadequate.
Repair
Once the correct diagnsos is made, the surgeon must act with celerity. Uterine replacement can be surprisingly easy when performed early and avoid the resort to the various and potentially morbid manipulations necessary to return the uterus to its correct anatomic position. As reviewed below, a potential exception to this rule of immediate replacement is the situation of inversion when the placenta remains attached to the uterus.
The best technique for rapid uterine replacement is controversial. In theory, manipulating the genital organs and restretching the pelvic viscera in the presence of some degree of neurologic shock could potentiate shock. As a practical matter, however this risk is inconsequential. Long clinical experience indicates that the best management is always rapid manual replacement, preferably by vagina manipulation. The longer the delay, the greater the risk for blood loss and maternal cardiovascular collapse and its serious sequelae. Also, as the delay lengthens, the lower uterine segment and/or cervix increasingly contracts, rendering the prolapsed fundus more edematous than before. Thus, delay simply makes replacement progressively more difficult.
The following steps are recommended:
Manually replace uterus (techniques discussed below). An above and below procedure is recommended
Suture lacerations of the birth canal and any surgical incisions in the cervix or vagina.
Perform uterine massage after replacement of the uterus.
Administer uterotonics. These drugs may include one of the following:
-
Methyl ergonovine maleate (Methergine; Novartis Pharmaceuticals Corporation, East Hanover, NJ) 0.2 mg given intramuscularly every 30 minutes for up to 3 times, as needed
-
Oxytocin 40-60 IU/L in an isotonic, balanced salt solution (eg, Ringer lactate) given as a constant infusion
-
Prostaglandin 15-methyl F2 alpha (carboprost tromethamine, Hemabate; Pharmacia & Upjohn Company, Division of Pfizer, New York, NY) 0.25 mg given intramuscularly every 30 minutes up to 3 times, as needed
-
Misoprostol 0.4 mg orally or buccally every 2 hours as needed, or, 0.8-1.0 mg per rectum given once
Closely monitor the patient for several hours after the uterus is replaced to detect spontaneous reinversion.
Because most deliveries now take place in labor/delivery/recovery rooms, this site is probably the most frequent venue for trials of immediate replacement if the placenta has separated. However, depending on the circumstances and on whether the placenta remains attached, a reasonable delay may be prudent. During this time, help is recruited (eg, anesthesiologist, nurses, surgical assistants, etc. ), laboratory tests are ordered, assistance from the blood bank is ensured, aggressive fluid resuscitation is started, and the parturient is transported to an operating room. There, the uterine inversion may be reduced by means of the traditional vaginal approach or a surgical procedure, as described later.
Nonsurgical replacement techniques
There are nonsurgical methods for replacement discussed in the literature. In 1945, O'Sullivan described a method for correcting partial inversion by using simple hydrostatic pressure. [76] In this technique, warm sterile water or isotonic sodium chloride solution is rapidly instilled into the vagina through a tube while the introitus is blocked with either an instrument (eg, vacuum extractor) or the obstetrician's forearm or fist. The instilled fluid progressively distends the vaginal wall and forces the fundus upward to restore it to its original position. Some clinicians favor a trial of this procedure in selected patients because of its simplicity, but it is not a popular method. [77, 78, 79]
Transvaginal Uterine Replacement
With the advent of potent tocolytics, the technique of manual replacement has been greatly simplified and a variant of the manipulations Johnson originally described in 1949 is currently the treatment of choice. [80] In this procedure, after the administration of the tocolytic and an analgesic, the operator's hand is placed in the vagina, with the palm cupping the inverted fundus. The uterus is then firmly and promptly lifted upward in the pelvic curve through the pelvis and into the abdominal cavity to the level of the umbilicus. This manipulation forces the uterine ligaments to stretch. When the inverted mass is pushed upward, the uterus typically reverts promptly, and the fundus returns to its anatomic position. If reversion is successful, the uterus is held in place for several minutes. Parenteral uterotonics are then administered to firm the myometrium and attention is then given to placental removal if the separation has not already occurred.
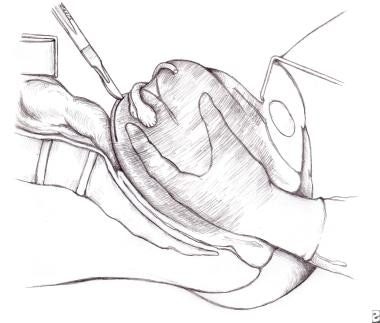 Uterine inversion. Image depicts the technique of manual replacement. Note the potential incision of the posterior uterine wall if surgical replacement is required.
Uterine inversion. Image depicts the technique of manual replacement. Note the potential incision of the posterior uterine wall if surgical replacement is required.
To facilitate uterine replacement, terbutaline, nitroglycerin, and, less frequently, magnesium sulfate have been successfully used as uterine relaxants with or without concomitant general anesthesia. In the period immediately following successful replacement, doses of uterotonics are serially administered to prevent reinversion, offset secondary atony, and control hemorrhage.
If the placenta has not separated before the replacement operation is attempted, the most prudent approach is to leave it undisturbed until the patient is in the operating room and an anesthetic or tocolytic is given. Immediate removal of the placenta without successful uterine replacement simply increases blood loss. Furthermore, in the uncommon event of placenta accreta, placenta increta, or placenta percreta, removal proves either incomplete or impossible and the efforts at removal markedly increase blood loss while valuable time is lost.
Unfortunately, the degree of abnormal placental adherence cannot be established until removal is attempted, and then it is too late to avoid a sudden and severe hemorrhage. Therefore, if inversion occurs and the placenta is still attached, the best plan is to wait for the relatively safe environment of the operating room rather than attempt an immediate and perhaps incomplete placental removal in an ill-equipped setting. Exceptions to this general rule exist in clinical settings when access to equipment or surgical assistance is limited.
Surgical techniques
The literature contains a number of possible alternative approaches to surgical replacement if transvaginal replacement fails. These include transabdominal and transvaginal methods including traditional surgical transperitoneal procedures as well as hydroscopic and laparocopic techniques. [81] As a practical matter, only the Huntington (laparatomy / traction from above) and Haultain (laparatomy / incision of ring and traction ) procedures are recommended. As a general rule, if 2 or more attempts at manual replacement are unsuccessful despite adequate tocolysis and analgesia, a surgical procedure is indicated. As noted, an abdominal approach for uterine replacement is favored. A trans vaginal technique has also been described, but it has few adherents.
In the vaginal procedure, the bladder is dissected from the cervix, and the anterior lip of the cervix and the anterior wall of the uterus are incised to the extent necessary to permit replacement. After the uterus is repositioned, the uterine wall and cervical defects are repaired in layers. Such transvaginal procedures are specifically not recommended except when done by especially trained surgeons or by those working under the immediate supervision of a properly experienced surgeon.
The favored transabdominal technique is a modification of the procedure Huntington originally described in 1921. [82] Deep general anesthesia is first induced with an agent that promotes uterine relaxation. A laparotomy is performed though a midline incision. The inversion site is marked by the peculiar sight of the round ligaments passing down into the pelvis at the anatomic site normally occupied by the uterus.
To perform inversion, the surgeon grasps the round ligaments or the myometrium with atraumatic clamps (eg, Babcock clamp) and applies gentle upward traction. The operator pulls the round ligament up into the peritoneal cavity, and, as the ligament advances, it is immediately grasped more distally with a second clamp. This process is akin to pulling on a rope, hand over hand. This maneuver is repeated alternatively on one side and the other until the fundus is completely restored to it normal configuration.
If available, a second operator facilitates the procedure by applying upward pressure from below. As the uterus begins to revert, the lower segment can be squeezed like a tube of toothpaste to accelerate the process. As in the manual replacement technique, uterotonics are administered as soon as the uterus has returned to its normal shape.
In the unusual instance that the Huntington operation is unsuccessful, the more extensive Haultain procedure is required. [83] This is usually necessary only when inversion is chronic or when tocolysis fails to adequately relax the lower segment. With this technique, a posterior longitudinal hysterotomy incision is performed. This widens the lower uterine segment and facilitates replacement of the uterus. The posterior uterine wall is incised to avoid inadvertent injury to the bladder that might occur with an anterior approach. Thereafter, upward traction is applied to the round ligaments, as in the Huntington procedure, until the uterus resumes its normal position. The myometrial defect is then repaired in layers. Once the uterus has been replaced, the placenta is removed. If an accreta or one of the more severe forms of placental adherence is present, the best approach is hysterectomy.
Regardless of the procedure used, immediate uterine atony is common after repositioning, and prompt reinversion may occur. Close observation for this complication is mandatory. Also recommended is the administration of 15-methyl F2alpha prostaglandin (Hemabate; Pharmacia & Upjohn Company), high-dose oxytocin, parenteral methylergonovine maleate (Methergine; Novartis Pharmaceuticals Corporation), or misoprostol per rectum. If magnesium sulfate was administered as a tocolytic, calcium can be administered parenterally to reverse the tocolytic effect. Subsequently, the correct positioning of the uterus can be verified by intermittent ultrasound scanning or by gentle palpation of the fundus.
The administration of antibiotics has been advocated when inversion occurs. This guidance is based on the theory that the various manipulations for replacement predispose the patient to develop an infection. The risk is probably small, but it is unknown. Many clinicians administer prophylactic doses of a broad-spectrum, first-generation cephalosporin or a similar drug after repositioning. No data concerning the need for such treatment are available. The decision to reat this condition or not is left to the discretion of the senior surgeon.
Inversion in nonpregnant women
Inversion is rarely observed in nonpregnant women. [45, 84] The characteristic finding in these most unusual patients is a pedunculated subserosal leiomyoma or other neoplasm at or adjacent to the uterine fundus. The usual culprits are endometrial polyps and pedunculated leiomyomata.
In these gynecologic cases, the mechanism for inversion is presumed to be similar to that which occurs postpartum, although the interval needed for inversion is apparently prolonged. The tumor or mass descends into the lower segment of the uterus. The uterus then responds with recurrent contractions. This drives the mass down and, on occasion, entirely through the cervix. When possible, the tumor should be excised and the uterus replaced with definitive treatment necessarily awaiting histologic examination and tissue diagnosis. Treatment with antibiotics is prudent to allow inflammation to resolve with a definitive procedure performed at a later time. Due to necrosis, edema and infection delay may not be possible. Final definitive treatment may involve vaginal hysterectomy or a combined above and below procedure. Because of the variation in tissue diagnosis and differences in presentation, individualization of case management is essential. Pulmonary embolism is associated with chronic or neglected casespresumably because of edema and secondary infection. Thus, in such instances, prophylaxis against thrombosis should be considered.
Recurrence, morbidity, and counseling
Recurrent inversion in subsequent deliveries does occur. The incidence is believed to be low, but it is fair to say that it is not accurately known. The historical series of 172 cases described by van Vugt included references to a number of previous reports in which recurrences were reported. No incidence figures were provided, however. In the 2002 report by Baskett of 40 cases of postpartum inversion drawn from 125,081 births over 24 years, no recurrences were recorded in the 26 subsequent deliveries. [63]
Maternal mortalities do occur in association with inversion but are usually restricted to neglected or complicated cases. As fatal cases are at best uncommon in Western medical practice, an accurate mortality risk from inversion cannot be calculated. Fatal cases are, however, still reported from the nonindustralized world.
Time is an important factor in maternal mortality. In van Vugt's series derived from cases over many years, mortality was lowest when the condition was recognized promptly (within 30 min of delivery) and treatment was begun within 2 hours postpartum. [85] With delay, mortalities were much more frequent. These facts emphasize the importance of celerity on the part of the birth attendants in establishing the diagnosis, replacing the uterus, and aggressively supporting the mother's condition.
If a surgical procedure with hysterotomy incision(s) were required for uterine replacement, the woman should be counseled that a risk of uterine rupture in a subsequent gestation during labor is possible. In theory, if the incision in myometrium remains in the lower uterine segment, the rupture risk should approximate that for the usual anterior vertical hysterotomy incision occasionally used for cesarean delivery. The actual rupture risk is unknown due to the absence of data.
Uterine inversion in animals and genetic factors
Uterine inversion is a relatively frequent problem in veterinary medicine. It is particularly dangerous and difficult to resolve among bovines.
A chance observation in mice by Yokoi and coworkers hints at the possibility of a genetic propensity for inversion that might have implications for human medicine. In specially bred mice, females homozygous for a gene locus that codes for deficiencies in melanocytes, mast cells, and osteoclasts had a remarkably high incidence of postpartum uterine inversion (86%). In some of these mice, the uterus was inverted with the placenta still implanted. [86]
After a series of subsequent cross-mating studies, the maternal genotype (not that of either the father or the fetus) was determined to be the important component in coding for this inversion risk. The results of this unusual experiment hinted that specific genes code for abnormal uterine tone during the immediate puerperium. Thus, an apparent functional disorder of the myometrium predisposes the individual rodents to develop puerperal inversion by means of an unknown mechanism. These data open the possibility of genetic predisposition as a new area for future investigation.
Uterine Prolapse
Frequency
The incidence of uterine prolapse during pregnancy is not accurately known. [87, 49, 88] Only case reports are available in the literature. All reviewers concur that this is a highly uncommon condition in the United States. More cases occur in obstetric practice in the developing world, where advanced multiparity and obstetric injuries are relatively common. By contrast, in gynecologic practice, prolapse is a common condition, especially among multiparous menopausal women.
While all reviewers agree that uterine prolapse during gestation is a markedly infrequent clinical problem moderate degrees of descensus are common before pregnancy, especially in multiparas. In most instances, pregnancy-associated prolapse partially or completely resolves in the midtrimester as the fundus grows and the uterus becomes an abdominal organ, drawing the cervix upward. In such cases, symptomatic prolapse usually recurs in the third trimester, or, on occasion, it is first observed at this time. Treatment is often not required except in the more extreme cases.
Pathophysiology
The female pelvic viscera are best considered to be suspended from above and supported from below. To maintain the pelvic organs in their proper position there are several suspensory and support structures. Continued support depends upon the integrity of the muscular, fascial, and neurologic components of various pelvic tissues. Substantial injury to one or more of these structures can result in a loss of support and result in a degree of prolapse that may prove permanent. [89, 90, 91, 92]
A complex of musculoconnective tissue forms a web of support for the pelvic organs. The upper suspensory structures for the uterus are a complex of pseudoligamentous tissues that are traditionally and loosely termed the pelvic ligaments. These sheets of connective tissue accompany vascular structures into the pelvis and surround the cervix and other pelvic structures. Included among these suspensory connective tissues are the pubovesical, cardinal, and uterosacral ligaments. In contrast to the upper tissues, the lower supports for the uterus are musculofascial. These include the urogenital diaphragm and the pelvic diaphragm. The latter principally consists of the several named divisions of the levator ani muscle. The urogenital diaphragm is a complex of muscular structures and accompanying connective tissue that radially extends from the central perineal body to attach to various bony and ligamentous sites in the pelvis.
A number of life events and conditions result in both reversible and permanent injuries to the maternal pelvic supports. These include dystocia in labor, vaginal delivery, obstetric lacerations, multiparity, age, genetic propensity, and, the mode of delivery (i.e. vacuum extraction or forceps vs. spontaneous, uninstrumented) . Passage of the baby through the birth canal is believed to produce most of the damage. Iimportant pelvic connective tissues are often simply torn, lacerated, denervated, or otherwise disrupted by the process of parturition. Descent of the presenting part elongates the levator ani muscle complex by up to 50% and traumatizes the pelvic nerves by stretch and direct pressure. Various spontaneous lacerations or episiotomy extensions account for additional injuries to perineal structures. The issue is not whether vaginal delivery results in injuries to the pelvic support tissues. The question is the degree of the injury and the extent to which spontaneous postpartum healing or specificmuscle-strengthening exercises performed in the puerperium may ameliorate this damage.
The etiology of uterine prolapse during pregnancy is multifactorial. It requires a combination of previous traumatic injuries to the upper fascial suspensory system, damage to the muscular supporting structures of the pelvic diaphragm, and a degree of neurogenic myopathy or nerve injury at various levels. These injuries are usually combined with overstretching or prior laceration of the musculoconnective tissues of the pelvic floor.
Although multiparity, dystocia, instrumental delivery, and lacerations of the birth canal are routine obstetric events, uterine prolapse during pregnancy is notable for its rarity. This observation indicates that some combination of pregravid structural injury to the support and suspension system, constitutional or hereditary weaknesses in the connective tissues, and the pressure and hormonal effects of an established pregnancy must occur to result in symptomatic gestational decensus.
Clinical Presentation
Signs and symptoms associated with uterine prolapse may include the following:
-
Pelvic pressure or discomfort
-
Lower back pain
-
Urinary tract infection
-
Paradoxical urinary tract symptoms (eg, acute retention, incontinence)
-
Preterm membrane rupture
-
Preterm labor
-
Cervicitis, vaginitis, vaginal discharge
-
Bleeding due to mucosal ulcerations or cervicitis
-
Vaginal or perineal mass
-
Constipation
Diagnosis
Establishing the diagnosis is not a challenge. On examination, the clinician observes an unusually low position of the cervix. This is often accompanied by variable protrusion of the anterior and posterior vaginal walls. In advanced cases, inflammation of the cervix, and occasionally even ulceration of the vaginal mucosa, is present. The protrusion of the cervix is partially ameliorated when the gravida assumes a horizontal position.
Uterine distortion due to tumors such as large leiomyomas or other masses, prolapse of a cervical or endocervical tumor, or a müllerian anomaly must be considered in the differential diagnosis when an abnormally low position of the cervix is noted.
Results from physical examination and ultrasonographic study will promptly establish the correct diagnosis.
Treatment
Aspects of treatment are as follows:
-
Restriction of activity.
-
Aggressive treatment of cervicitis or soft tissue erosions of the birth canal.
-
In selected cases, a trial of a pessary.
-
Treatment of associated urinary tract infections.
-
In early gestation, administration ofr corticosteroids to enhance fetal pulmonic maturity.
-
Serial assessment of fetal growth
-
Sonographic assessment of fetal anatomy.
-
Biochemical testing to evaluate for aneuploidy risk
Although treatment with a pessary may be successful early in gestation, it usually fails by the onset of the third trimester due to laxity of the pelvic tissues and the unrelenting pressure of the gravid uterus. Thereafter, restriction of activity becomes the principal means of management, along with symptomatic treatment for cervicitis or urinary tract infection and close clinical observation for obstetric complications. Close clinical observation in this situation is mandatory.
Prolapse is uncommon, but an unfortunate and all-too-common associated problem is the high incidence of obstetric complications. These are principally complications related to premature rupture of the membranes, preterm labor, and, eventually, preterm delivery.
The appropriate postpartum recommendation is unclear. For symptomatic decensus a pessary may be tried. Patient counseling, interim contraception, sterilization, and hysterectomy with vault suspension are to be considered. Successful pregnancies after mesh-supported uterine suspensions have been reported, but the numbers are few.
Serious complications
A number of serious complications are reported in association with prolapse during pregnancy. However, the number of cases is small, and they do not represent the entire population of women with this disorder. After pregnancy ends, symptomatic prolapse may confidently be anticipated to recur in these women. Again, firm data on this point are not available.
Among nonpregnant women, hysterectomy with vault suspension is usually required to definitively repair advanced degrees of prolapse. The appropriate procedure depends on the severity of the problem and on the individual's pelvic anatomy.
Possibility of vaginal delivery
A surprising observation is that vaginal delivery is often possible in cases of pregnancy-related prolapse. However, a word of caution is appropriate. A possible vaginal trial must be reevaluated if severe cervicitis or edema is present, especially when insufficient time remains to permit adequate antepartum treatment.
Cervical inflammation and its treatment
In advanced cases cervical inflammation is an invariable finding. The biochemical effects of infection and the resultant inflammatory response are possibly etiologic in the oft-reported complications of membrane rupture and preterm labor. Prompt and adequate treatment of cervicitis and close attention to any concomitant urinary tract infection are prudent. However, treatment of these infections does not invariably reduce the risk of premature rupture of membranes or preterm labor.
In the common condition of midtrimester bacterial vaginosis and cervicitis, antibiotic therapy has minimal effect on preventing prematurity or early membrane rupture. By the time the severe inflammation that characterizes a prolapsed cervix during pregnancy develops, the various promoters of early inappropriate uterine activity may already been elaborated. If so, they render antibiotic therapy ineffectual in the prevention of prematurity.
Other management steps may be taken. Testing for fetal fibronectin helps in estimating the risk of early delivery, which can alert both the patient and the clinician that the immediate risk of preterm delivery is increased. If prolapse is diagnosed after the 24th week and before the 33rd week, steroid treatment to enhance fetal lung maturity should also be strongly considered.
Other than use of a pessary, treatment of cervicitis or urinary tract infection, and activity restriction, a possible future treatment for pregnant women with prolapse might be the parenteral or intravaginal administration of progesterone.
Data indicate that prophylactic progesterone therapy reduces the incidence of preterm labor. Once labor ensues, this treatment is ineffective. Therefore, patients at risk must be identified, and therapy should be started before the signs and symptoms of labor begin. At present, no data address the effectiveness of progesterone therapy in pregnant women with uterine prolapse and cervicitis. Treatment is left to the clinician's discretion.
-
Uterine retroversion and incarceration progressing to sacculation as pregnancy advances.
-
Uterine retroversion-incarceration has occurred. Depicted is the technique of manual uterine replacement with the patient in the knee-chest position.
-
Uterine inversion. Image depicts the technique of manual replacement. Note the potential incision of the posterior uterine wall if surgical replacement is required.